 2022 Annual Clinical & Scientific Meeting
2022 Annual Clinical & Scientific MeetingIn Utero Enzyme Replacement Therapy
Phase 1 Clinical Trial: In Utero Enzyme Replacement Therapy (ERT) for Fetuses with Lysosomal Storage Diseases (LSDs)
Contact the study team: fetaltreatmentcenter@ucsf.edu or 1-800-RX-FETUS
Overview
Lysosomal storage diseases (LSDs) are inborn errors of metabolism that are caused by a mutation coding for a critical enzyme. This causes an abnormal build-up of toxic materials throughout the body. LSDs cause severe multi-organ damage, including neurodegeneration, cardiac problems, impaired growth, and enlarged spleen and liver. While each LSD is a rare disease, collectively LSDs affect 1 in 5000 births.
This is a phase 1 clinical trial of in utero (meaning before birth) enzyme replacement therapy for fetuses with certain LSDs. The purpose of this study is to test the safety (to the fetus and the mother) and feasibility (meaning how easy is it to do) of enzyme replacement therapy in fetuses with these diseases. Standard care for these diseases involves giving enzyme replacement therapy (ERT) after birth. We believe that the potential benefits of giving ERT before birth are the following:
- After children receive ERT multiple times, they can develop antibodies against the medicine because their bodies start seeing it as a foreign protein. We think that the unique immune system of the fetus could result in the fetus not seeing the enzyme as foreign. This could prevent the development of antibodies and/or an allergic reaction to the medicine.
- Enzyme replacement therapy cannot get into the child’s brain and thus cannot treat any effects of the disease on the brain. Enzyme given before birth can cross into the brain because of the fetus’ developing blood brain barrier. Giving ERT before birth may prevent or slow the progression of the disease on the brain.
- Lysosomal storage diseases can start causing damage to a patient before birth or shortly after birth. Some fetuses do not survive to birth and thus do not receive ERT. By giving the ERT before birth, we hope to improve the chances of the fetus surviving to birth and prevent the disease from starting to cause damage.
This is the first time in utero enzyme replacement therapy will be performed in humans. Previous studies in our lab were done in mice with a lysosomal storage disease, which showed that giving ERT before birth improved survival, decreased the effect of the disease on different parts of the body, and promoted tolerance (meaning the body did not recognize the enzyme as foreign and did not develop antibodies or an allergic reaction) compared to mice who only received ERT after birth.
Diseases included in this trial:
- Mucopolysaccharidosis type 1
- Mucopolysaccharidosis type 2
- Mucopolysaccharidosis type 4a
- Mucopolysaccharidosis type 6
- Mucopolysaccharidosis type 7
- Infantile-onset Pompe disease (IOPD)
- Neuronopathic Gaucher disease (types 2 and 3)
- Wolman disease
Trial design
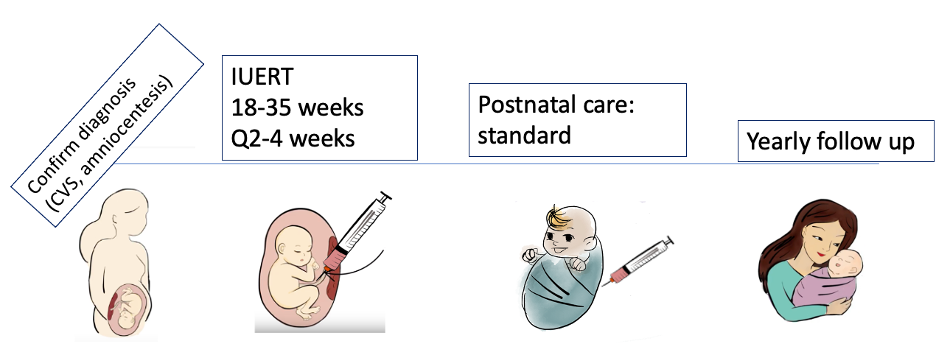
We will enroll 10 women with a fetus diagnosed with one of the included LSDs, based on chorionic villus sampling (CVS) or amniocentesis. Between 18 to 35 weeks gestation, we will give the weight-based dose of the relevant FDA-approved enzyme replacement therapy every 2-4 weeks. This will be done by an umbilical vein injection, which is a procedure routinely used for in utero blood transfusions, and that has a good safety profile. After birth, the baby will receive standard care, including ERT and possibly stem cell transplantation (depending on the disease). We will follow the child for 5 years after birth, to determine whether in utero ERT improves long-term outcomes such as neurologic function, mobility, and growth.
Trial Aims
- Determine safety of in utero ERT to the fetus and the mother
- Determine feasibility of identifying and treating fetuses with in utero ERT
- Determine whether in utero ERT reduces the accumulation of toxic metabolites (glycosaminoglycans)
- Determine whether in utero exposure to recombinant enzyme induces tolerance
Educational Information
Clinicaltrials.gov Identifier: NCT04532047
Principal Investigator: Tippi C. MacKenzie, MD